Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ishijima, Yasuhiro; Ueno, Fumiyoshi; Abe, Hitoshi
Materials Transactions, 63(4), p.538 - 544, 2022/04
Times Cited Count:0 Percentile:0(Materials Science, Multidisciplinary)The time dependence of the corrosion behavior of tantalum (Ta), which is used in nuclear fuel reprocessing equipment, in sodium hydroxide (NaOH) solutions was investigated by immersion tests, and the mechanism of the time dependence was examined via surface observations and electrochemical measurements. The immersion tests were conducted at room temperature with NaOH concentrations ranging from 1 to 7 mol/L for immersion periods of 24 to 168 h. The corrosion rate increased with the NaOH concentration but peaked with the immersion period and then decreased. The time to peak of the corrosion rate was shorter with higher NaOH concentration. The X-ray diffraction (XRD) patterns and Raman spectra of the surfaces of the specimens immersed in the 7 mol/L NaOH solution for more than 48 h showed Na Ta
Ta O
O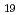 formation. The polarization resistance decreased with immersion time for all NaOH concentrations up to about 24 h after immersion. Thereafter, the polarization resistance increased with immersion time in 7 mol/L NaOH solution and remained almost constant in the other NaOH concentrations. Findings suggested that the change in the corrosion rate was affected by the film formation during immersion, since the time dependence of the polarization resistance and the sum of film resistance and charge transfer resistance had the same tendencies. The precipitation film was mainly Na
formation. The polarization resistance decreased with immersion time for all NaOH concentrations up to about 24 h after immersion. Thereafter, the polarization resistance increased with immersion time in 7 mol/L NaOH solution and remained almost constant in the other NaOH concentrations. Findings suggested that the change in the corrosion rate was affected by the film formation during immersion, since the time dependence of the polarization resistance and the sum of film resistance and charge transfer resistance had the same tendencies. The precipitation film was mainly Na Ta
Ta O
O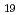 formed by the dissolution of the passivity film on Ta.
formed by the dissolution of the passivity film on Ta.
Ishijima, Yasuhiro; Ueno, Fumiyoshi; Abe, Hitoshi
Zairyo To Kankyo, 70(6), p.192 - 198, 2021/06
The time dependence of corrosion behavior on tantalum used in nuclear fuel reprocessing equipment in sodium hydroxide solution was investigated by immersion corrosion tests, and the mechanism of aging change was discussed from surface observations and electrochemical measurements. The immersion tests were carried out at room temperature with NaOH concentrations ranging from 1 to 7 mol/L and immersion times ranging from 24 to 168 hr, respectively. The corrosion rate increased with NaOH concentration, but peaked with immersion time and then decreased. The time to peak of corrosion rate was shorter with higher NaOH concentration. The SEM observations and Raman analysis at the surface of the specimens that were cleaned and weighed after the immersion test did not show any film formation. On the other hand, the polarization resistance showed a constant value or an increase after a decrease immediately after immersion. It is suggested that the change in corrosion rate is affected by the formation of film by immersion, since the value of polarization resistance is almost the same as the sum of film resistance and charge transfer resistance. The film was considered to be mainly Na Ta
Ta O
O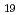 formed by the dissolution of Ta.
formed by the dissolution of Ta.